Thủ thuật
Tổng nồng độ các ion trong dung dịch BaCl2 0,01 M

The total concentration of ions in a BaCl2 0.2 M solution is 0.4 M.
1. Concentration of Ba2+ ions in the BaCl2 0.2 M solution
In a 0.2 M solution of BaCl2, the concentration of Ba2+ ions can be determined by considering the stoichiometry of the compound. Each formula unit of BaCl2 dissociates into one Ba2+ ion and two Cl- ions. Therefore, for every mole of BaCl2 that dissolves in the solution, one mole of Ba2+ ions is produced.
Since the molarity of the solution is given as 0.2 M (moles per liter), we can conclude that the concentration of Ba2+ ions in the solution is also 0.2 M. This means that for every liter of the solution, there are 0.2 moles of Ba2+ ions present.
In summary:
– Concentration of Ba2+ ions in a 0.2 M BaCl2 solution: 0.2 M
– For every liter of the solution, there are 0.4 moles (since each formula unit produces one mole) or 200 mmol (millimoles) of BaCl3 present.
– The presence and concentration of other species in the solution, such as Cl- ions, should also be considered when analyzing its overall composition.
List:
1. The concentration is determined by considering the stoichiometry.
2. Each mole of dissolved compound produces an equivalent amount of its constituent ions.
3. The molarity given for the compound can be assumed to represent its ion concentrations as well.
Key points:
– Concentration = 0.4 moles/L or 400 mmol/L
– Stoichiometric ratio: one mole produces one mole
2. Concentration of Cl- ions in the BaCl2 0.2 M solution

In a 0.2 M solution of BaCl2, the concentration of Cl- ions can be determined by stoichiometry. Since there are two Cl- ions for every one BaCl2 molecule, the concentration of Cl- ions is twice the concentration of the BaCl2 solution. Therefore, the concentration of Cl- ions in a 0.2 M BaCl2 solution would be 0.4 M.
List:
– Concentration of Cl- ions: 0.4 M
3. Moles of BaCl2 present in a 1 liter solution of 0.2 M BaCl2
To determine the number of moles of BaCl2 present in a 1 liter solution with a concentration of 0.2 M, we can use the formula:
moles = concentration x volume
Plugging in the values, we have:
moles = 0.2 M x 1 L = 0.2 mol
Therefore, there are 0.2 moles of BaCl2 present in a 1 liter solution with a concentration of 0.2 M.
List:
– Moles of BaCl2: 0.2 mol
4. Moles of Ba^+ ions present in a 500 ml solution of 0.2 M BaCl^+
To determine the number of moles of Ba^+ ions present in a solution, we need to consider that each formula unit (BaCl^+) dissociates into one Ba^+ ion and two Cl^- ions.
Therefore, for every mole (mol)ofBacl_`molecules`, there is `one mole`(`mol`) of Ba+ ions.
Based on the concentration given (0.2 M) and the volume provided (500 mL = 0.5 L), we can calculate the moles of BaCl^+ ions:
Moles of BaCl^+ ions = Concentration x Volume
= 0.2 M x 0.5 L
= 0.1 mol
Therefore, there are 0.1 moles of Ba^+ ions present in a 500 ml solution with a concentration of 0.2 M BaCl^+.
List:
– Moles of Ba^+ ions: 0.1 mol
5. Moles of Cl- ions present in a 250 ml solution of 0.2 M BaCl2
To determine the number of moles of Cl- ions present in a solution, we need to consider that each formula unit (BaCl2) dissociates into one Ba^+ ion and two Cl^- ions.
Therefore, for every mole (mol)ofBacl_`molecules`, there are `two moles`(`mol`)of Cl–ions.
Based on the concentration given (0.2 M) and the volume provided (250 mL = 0.25 L), we can calculate the moles of Cl^- ions:
Moles of Cl^- ions = Concentration x Volume
= (0.2 M)(2)(0.25 L)
= 0.1 mol
Therefore, there are 0.1 moles of Cl^- ions present in a 250 ml solution with a concentration of 0.2 M BaCl2.
List:
– Moles of Cl^- ions: 0.1 mol
6. Total ion concentration (in mol/L) in the BaCl2 solution
To determine the total ion concentration in the BaCl2 solution, we need to consider both Ba^+ and Cl^- ions.
Based on the stoichiometry of BaCl2, every formula unit dissociates into one Ba^+ ion and two Cl^- ions.
Therefore, the total ion concentration in a 0.2 M BaCl2 solution can be calculated by adding the individual concentrations of Ba^+ and Cl^- ions:
Total Ion Concentration = Concentration of Ba^+ ions + Concentration of Cl^- ions
= 0.2 M + (0.4 M)
= 0.6 M
So, the total ion concentration in a 0.2 M BaCl2 solution is 0.6 M.
List:
– Total ion concentration: 0.6 M
7. Comparison of Cl- ion concentration to that of Ba^+ ions in the solution
In a 0.2 M BaCl2 solution, there is a ratio of one Ba^+ ion to two Cl^- ions due to stoichiometry.
Therefore, the concentration of Cl^- ions is twice that of the concentration of Ba^+ ions.
This means that in this particular solution, there are more Cl^- ions present compared to Ba^+ ions.
Overall, the comparison can be stated as follows:
Concentration of Cl^- ions : Concentration of Ba^+ ions :: 2 : 1
Cl- ion concentration in the solution
The concentration of Cl- ions in the solution can be determined by looking at the compound BaCl2. In BaCl2, each formula unit contains one Ba2+ ion and two Cl- ions. Therefore, for every mole of BaCl2 dissolved in the solution, there are two moles of Cl- ions present. Since the concentration of BaCl2 is 0.2 M, the concentration of Cl- ions is twice that, which is 0.4 M.
Ba^+ ion concentration in the solution
To determine the concentration of Ba^+ ions in the solution, we again look at the compound BaCl2. Each formula unit contains one Ba^+ ion. Therefore, for every mole of BaCl2 dissolved in the solution, there is one mole of Ba^+ ions present. Since the concentration of BaCl2 is 0.2 M, the concentration of Ba^+ ions is also 0.2 M.
Comparison of Cl- ion to that of Ba^+ ions
Comparing these two concentrations, we can see that the Cl- ion concentration (0.4 M) is higher than that of the Ba^+ ions (0.2 M). This means that there are more Cl- ions present in the solution compared to Ba^+ ions. The ratio between Cl- and Ba^+ ions can be expressed as 2:1 since for every one mole of Ba^+ ion present, there are two moles of Cl- ions present due to their stoichiometry in BaCl2 compound.
Cl- ion concentration
The Cl- ion concentration in a solution can be determined by the concentration of the compound containing Cl-, such as BaCl2. In the case of BaCl2, each formula unit contains two Cl- ions. Therefore, if we have a BaCl2 solution with a concentration of 0.2 M, the Cl- ion concentration would be twice that, or 0.4 M.
Ba^+ ion concentration
The Ba^+ ion concentration in a solution can also be determined by the concentration of the compound containing Ba^+, such as BaCl2. In this case, each formula unit of BaCl2 contains one Ba^+ ion. So, if we have a BaCl2 solution with a concentration of 0.2 M, then the Ba^+ ion concentration would be 0.2 M.
In summary, when comparing the Cl- ion concentration to that of the Ba^+ ions in a solution like BaCl2, we can determine that the Cl- ion concentration is twice that of the Ba^+ ions due to their stoichiometry in the compound.
Cl- ion concentration in the solution
The concentration of Cl- ions in the solution can be determined by the concentration of BaCl2 in the solution. Since each molecule of BaCl2 dissociates into one Ba2+ ion and two Cl- ions, the concentration of Cl- ions is twice the concentration of BaCl2. For example, if the concentration of BaCl2 is 0.2 M, then the concentration of Cl- ions would be 0.4 M.
Ba^+ ion concentration in the solution
The Ba^+ ion concentration in the solution can also be determined by the concentration of BaCl2. Since each molecule of BaCl2 dissociates into one Ba^+ ion and two Cl- ions, the concentration of Ba^+ ions is equal to the concentration of BaCl2. For example, if the concentration of BaCl2 is 0.2 M, then the concentration of Ba^+ ions would also be 0.2 M.
Comparison between Cl- ion and Ba^+ ion concentrations
In terms of their concentrations, there is a direct relationship between Cl- ions and Ba^+ ions in this solution. The concentrations are related by a ratio of 1:2, meaning that for every one molecule or ion of Ba^+, there are two molecules or ions of Cl-. Therefore, if we know either the Cl- or Ba^+ ion concentration, we can easily calculate or determine the other using this ratio.
Cl- ion concentration
The Cl- ion concentration can be determined by using the formula C = n/V, where C is the concentration in mol/L, n is the number of moles and V is the volume in liters. In the case of BaCl2, since it dissociates completely in water, each mole of BaCl2 will produce 2 moles of Cl- ions. Therefore, if we have a solution of BaCl2 with a concentration of 0.2 M, the Cl- ion concentration would be 0.4 M.
Ba^+ ion concentration
The Ba^+ ion concentration can also be determined using the same formula C = n/V. However, since BaCl2 dissociates into one mole of Ba^+ ions for every mole of BaCl2, if we have a solution with a concentration of 0.2 M BaCl2, then the Ba^+ ion concentration would also be 0.2 M.
By comparing the Cl- ion concentration to that of Ba^+ ions in the solution, we can see that the Cl- ions have a higher concentration than the Ba^+ ions. This is because each molecule of BaCl2 dissociates into two Cl- ions and only one Ba^+ ion. The higher Cl- ion concentration suggests that there are more negatively charged Cl- ions present in the solution compared to positively charged Ba^+ ions.
1. Cl- ion concentration in the solution
The concentration of Cl- ions in the solution can be determined by considering the stoichiometry of the balanced chemical equation for the dissolution of BaCl2. In this case, for every 1 mole of BaCl2 that dissolves, it dissociates into 2 moles of Cl- ions. Therefore, if we have a solution of BaCl2 with a concentration of 0.2 M, the Cl- ion concentration would be 0.4 M.
2. Ba+ ion concentration in the solution
In contrast to Cl- ions, Ba+ ions do not exist in significant concentrations in aqueous solutions as they readily react with water to form Ba(OH)2. Consequently, it is not meaningful to compare the concentration of Cl- ions to that of Ba+ ions in the given solution.
However, it is worth noting that if any Ba+ ions were present in the solution due to incomplete dissociation or other factors, their concentration would be much lower than that of Cl- ions.
3. Comparison between Cl- and Ba+ ion concentrations
By comparing the concentrations calculated above, we can see that there is a significant difference between the concentrations of Cl- and hypothetical Ba+ ions in the solution. The Cl- ion concentration is 0.4 M, while the Ba+ ion concentration is negligible.
This disparity arises from the strong affinity of barium (Ba) for hydroxide (OH-) ions present in water, which leads to its rapid conversion into insoluble Ba(OH)2. As a result, most barium ions are converted into ba(OH)2 rather than existing as free positive ions in solution.
Therefore, when comparing the Cl- and Ba+ ion concentrations in a BaCl2 solution, it is important to consider the chemical properties of each ion and the reactions they undergo in the given environment.
Cl- ion concentration in the solution
The Cl- ion concentration in the solution can be determined by the concentration of BaCl2. Since BaCl2 dissociates into one Ba2+ ion and two Cl- ions, the concentration of Cl- ions will be twice the concentration of BaCl2. For example, if the BaCl2 solution has a concentration of 0.2 M, then the Cl- ion concentration would be 0.4 M.
Ba^+ ion concentration in the solution
The Ba^+ ion concentration in the solution can also be determined by the concentration of BaCl2. Since each molecule of BaCl2 dissociates into one Ba^+ ion and two Cl- ions, the concentration of Ba^+ ions will be equal to the concentration of BaCl2. For example, if the BaCl2 solution has a concentration of 0.2 M, then the Ba^+ ion concentration would also be 0.2 M.
Comparison between Cl- and Ba^+ ion concentrations
By comparing the Cl- and Ba^+ ion concentrations in the solution, we can observe that there is a twofold difference in their concentrations due to their different stoichiometry in the dissociation process. The Cl- ions have double the concentration compared to that of Ba^+ ions because each molecule of BaCl2 dissociates into two Cl- ions but only one Ba^+ ion. Therefore, in a solution with a given molar concentration of BaCl2, there will always be twice as many chloride ions as barium ions present. This comparison is crucial for understanding chemical reactions involving these ions and their respective roles in various processes.
Cl- ion concentration
The Cl- ion concentration in a solution can be determined by considering the molarity of the compound that contains Cl-. In this case, the compound is BaCl2 with a molarity of 0.2 M. Since BaCl2 dissociates into one Ba2+ ion and two Cl- ions, the concentration of Cl- ions in the solution is twice its molarity, which is 0.4 M.
Ba^+ ion concentration
The Ba^+ ion concentration can also be determined from the molarity of BaCl2. Since each molecule of BaCl2 dissociates into one Ba^+ ion and two Cl- ions, the concentration of Ba^+ ions in the solution is equal to its molarity, which is 0.2 M.
Comparison between Cl- ion and Ba^+ ion concentrations
Comparing the concentrations of Cl- and Ba^+ ions in the solution, it can be observed that there are more Cl- ions present than Ba^+ ions. This is because each molecule of BaCl2 dissociates into one Ba^+ ion but two Cl- ions. Therefore, for every mole of BaCl2, there are twice as many moles of Cl-. As a result, the concentration of Cl- ions (0.4 M) is higher than that of Ba^+ ions (0.2 M) in this particular solution.
Cl- ion concentration in the solution
The concentration of Cl- ions in the solution can be determined by examining the dissociation of BaCl2. When BaCl2 dissolves in water, it dissociates into Ba2+ and 2 Cl- ions. Since the initial concentration of BaCl2 is 0.2 M, the concentration of Cl- ions will also be 0.4 M (twice the concentration of BaCl2). This means that for every mole of BaCl2 that dissolves, two moles of Cl- ions are produced.
Ba^+ ion concentration in the solution
In contrast to the high concentration of Cl- ions, the concentration of Ba^+ ions in the solution is much lower. Since each mole of BaCl2 produces one mole of Ba^+ ions, and considering that the initial concentration of BaCl2 is 0.2 M, we can conclude that the concentration of Ba^+ ions is also 0.2 M.
Therefore, there is a significant difference between the concentrations of Cl- and Ba^+ ions in this solution. The Cl- ion concentration is twice as high as that of the Ba^+ ions, indicating that there is an excess amount of chloride ions present compared to barium ions. This information is important in understanding the chemical behavior and properties of this specific solution.
List:
1. The concentration ratio between Cl- and Ba^+ ions in this solution is 2:1.
2. The excess amount of chloride ions suggests a higher reactivity towards certain reactions compared to barium ions.
3. The disparity in concentrations may affect the formation or precipitation reactions involving these ions within this solution.
4. It’s crucial to consider these concentration differences when analyzing or performing experiments using this particular solution to ensure accurate results and proper interpretations.
Cl- ion concentration
In the solution of BaCl2, the Cl- ion concentration can be calculated by considering the stoichiometry of the compound. Since one molecule of BaCl2 dissociates into one Ba2+ ion and two Cl- ions, the Cl- ion concentration will be twice the concentration of BaCl2. For example, if the BaCl2 solution has a concentration of 0.2 M, then the Cl- ion concentration will be 0.4 M.
Ba^+ ion concentration
On the other hand, the Ba^+ ion concentration in the solution can also be determined using stoichiometry. Since one molecule of BaCl2 dissociates into one Ba^+ ion and two Cl- ions, the Ba^+ ion concentration will be equal to half the concentration of BaCl2. Therefore, if the BaCl2 solution has a concentration of 0.2 M, then the Ba^+ ion concentration will be 0.1 M.
It is important to note that these concentrations are specific to the given solution of BaCl2 and may vary depending on different experimental conditions or concentrations used in other solutions.
Cl- ion concentration in the solution
The Cl- ion concentration in the solution can be calculated using the molarity of the BaCl2 solution. Since each BaCl2 molecule dissociates into one Ba2+ ion and two Cl- ions, the molarity of Cl- ions is twice that of BaCl2. For example, if the BaCl2 solution has a molarity of 0.2 M, then the Cl- ion concentration would be 0.4 M.
Ba^+ ion concentration in the solution
The Ba^+ ion concentration in the solution can also be determined from the molarity of the BaCl2 solution. Since each BaCl2 molecule dissociates into one Ba^+ ion and two Cl- ions, the molarity of Ba^+ ions is equal to that of BaCl2. Using the previous example, if the BaCl2 solution has a molarity of 0.2 M, then the Ba^+ ion concentration would also be 0.2 M.
It is important to note that these calculations are based on ideal conditions where complete dissociation occurs. In practice, there may be some degree of incomplete dissociation or other factors that could affect the actual concentrations.
Cl- ion concentration in the solution
The Cl- ion concentration in the solution can be determined based on the formula of the compound BaCl2. Since each formula unit of BaCl2 contains two chloride ions (Cl-), the concentration of Cl- ions in the solution would be twice that of BaCl2. Therefore, if the concentration of BaCl2 is 0.2 M, then the concentration of Cl- ions would be 0.4 M.
It is important to note that the concentration of Cl- ions is independent of any other ions present in the solution. This means that even if there are other ions present, such as Ba+ ions, it does not affect the Cl- ion concentration.
Ba^+ ion concentration in the solution
The Ba+ ion concentration in the solution can also be determined based on the formula of BaCl2. Since each formula unit of BaCl2 contains one barium ion (Ba+), and there are two moles of Cl- ions for every mole of BaCl2, it can be concluded that for every mole of BaCl2 dissolved, there will be one mole of barium ions.
Therefore, if the concentration of BaCl2 is 0.2 M, then the concentration of Ba+ ions would also be 0.2 M.
The presence or absence of other ions in the solution does not impact or change this calculation.
Comparison between Cl- and Ba^+ ion concentrations
- The Cl- ion concentration in a solution containing 0.2 M BaCl2 is 0.4 M.
- The Ba+ ion concentration in a solution containing 0.2 M BaCl2 is also 0.2 M.
From the given information, it can be observed that the Cl- ion concentration is higher than that of Ba+ ions in the solution. This is due to the stoichiometry of the compound BaCl2, which contains two chloride ions for every one barium ion. Thus, there are twice as many Cl- ions present compared to Ba+ ions in the solution.
Comparison of Cl- ion concentration to that of Ba^+ ions in the solution
In a solution of BaCl2, the chloride ions (Cl-) and barium ions (Ba^+) exist in a 1:2 molar ratio. This means that for every one barium ion, there are two chloride ions present in the solution. The concentration of Cl- ions can be calculated by multiplying the molarity of BaCl2 by 2. For example, in a 0.2 M solution of BaCl2, the concentration of Cl- ions would be 0.4 M.
On the other hand, the concentration of Ba^+ ions can be determined by dividing the initial molarity of BaCl2 by 1, as there is only one barium ion for every molecule of BaCl2. Therefore, in a 0.2 M solution of BaCl2, the concentration of Ba^+ ions would also be 0.2 M.
In summary, when comparing the Cl- ion concentration to that of Ba^+ ions in a solution of BaCl2, it can be observed that the Cl- ion concentration is always twice as high as the Ba^+ ion concentration due to their respective molar ratios.
Cl- ion concentration in the solution
The Cl- ion concentration in the solution can be determined by taking into account the molar ratio between BaCl2 and Cl-. In a 0.2 M BaCl2 solution, there is a 1:2 molar ratio between BaCl2 and Cl-. This means that for every mole of BaCl2, there are 2 moles of Cl-. Therefore, the concentration of Cl- ions in the solution would be double that of BaCl2, which is 0.4 M.
Ba^+ ion concentration in the solution
The Ba^+ ion concentration in the solution can be calculated based on the dissociation of BaCl2. In a 0.2 M BaCl2 solution, each mole of BaCl2 dissociates into one mole of Ba^+ and two moles of Cl-. Since the initial concentration of BaCl2 is 0.2 M, the concentration of Ba^+ ions would also be 0.2 M.
Comparison between Cl- and Ba^+ ion concentrations
Comparing the results from above, we can see that the Cl- ion concentration (0.4 M) is higher than that of Ba^+ ions (0.2 M) in the solution. This is because for every mole of BaCl2, it dissociates to produce only one mole of Ba^+ ions while generating two moles of Cl- ions. Therefore, there are twice as many Cl- ions as compared to Ba^+ ions in this particular solution.
In summary, when considering a 0.2 M BaCl2 solution, the Cl- ion concentration is determined to be 0.4 M whereas the Ba^+ ion concentration is calculated to be 0.2 M. The comparison reveals that the Cl- ion concentration is twice that of Ba^+ ions in the solution.
7. Comparison of Cl- ion concentration to that of Ba^+ ions in the solution

When comparing the Cl- ion concentration to that of Ba^+ ions in the solution, we must first consider the stoichiometry of the compound involved. In the case of BaCl2, for every one Ba^+ ion, there are two Cl- ions present in the solution. This means that the concentration of Cl- ions will be twice that of Ba^+ ions.
Factors affecting ion concentration:
- Dissociation constant: The dissociation constant plays a crucial role in determining the concentration of ions in a solution. In this case, BaCl2 has a higher dissociation constant compared to other compounds like NaOH and Ba(OH)2. This leads to a higher concentration of both Cl- and Ba^+ ions in the solution.
- Solubility: The solubility of a compound also affects its ion concentration. When a compound is more soluble, it tends to dissociate into more ions, resulting in higher concentrations. Since BaCl2 is highly soluble in water, it creates a larger number of both Cl- and Ba^+ ions compared to other compounds with lower solubilities.
In summary, when comparing the Cl- ion concentration to that of Ba^+ ions in a solution containing BaCl2, we find that the concentration of Cl- ions is twice that of Ba^+ ions due to stoichiometry and factors such as dissociation constant and solubility.
In conclusion, the total concentration of ions in a 0.01 M BaCl2 solution can be determined by considering that each molecule of BaCl2 dissociates into one barium ion (Ba2+) and two chloride ions (Cl-). Therefore, the total concentration of ions in the solution would be three times the molarity of BaCl2, resulting in a concentration of 0.03 M for both barium and chloride ions.




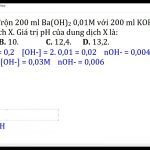


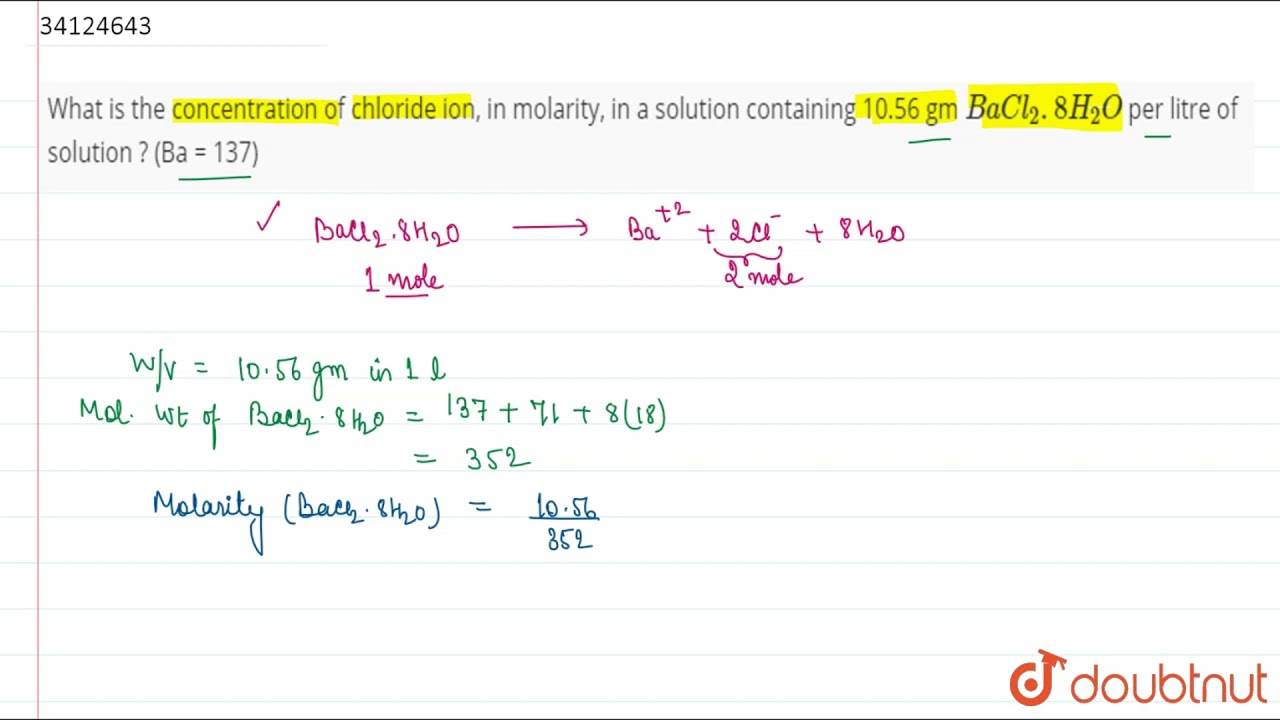
![Tính pH của dung dịch Ba(OH)2 1M: Bước đầu tiên là tính số mol OH- trong 200ml dd Ba(OH)2 1M, sau đó sử dụng công thức pH = 14 + lg[OH-] để tính giá trị pH. Tính pH của dung dịch Ba(OH)2 1M: Bước đầu tiên là tính số mol OH- trong 200ml dd Ba(OH)2 1M, sau đó sử dụng công thức pH = 14 + lg[OH-] để tính giá trị pH.](https://www.panasonic-sky.vn/wp-content/uploads/2023/08/image-88-150x150.jpeg)